PH Full Form | What is Potential of Hydrogen
What is the full form of PH
PH: Potential of Hydrogen
PH stands for Potential of Hydrogen. It refers to the hydrogen ion concentration in a solution. It is the measure of the acidity or alkalinity of a solution. The PH value ranges from 0 to 14 on a pH scale.
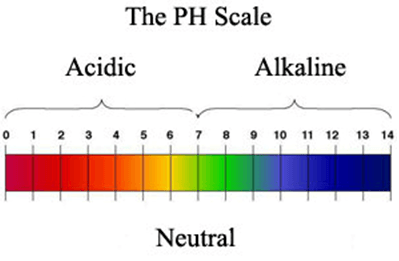
The PH values below 7 indicate acidity and values above 7 indicate alkalinity. Acidity increases as the PH value decreases and alkalinity increases as the PH value increases. A solution with PH 1 will be highly acidic and with PH 14 will be highly alkaline. A neutral solution has pH of 7, e.g. pure water at 25℃C has PH of 7.
Related Full Forms